
|
|
|
|
|
|
|
|
 |
|
|
|
||||
|
|
|
||||
     |
Reticle Carbon Capacitive Deionization Cells Reticle Carbon© prospectively allows a sea change in the economics and efficacy of a known, simple, electrostatic process for water purification known as capacitive deionization (CDI), which works as follows. If ion-bearing water were passed between two oppositely charged electrodes as illustrated in Figure 1, the negatively charged ions would be arrested or "adsorbed" on the anode by simple electrostatic attraction and the positively charged ions would be arrested on the cathode by simple electrostatic attraction. (In the case of brackish water containing salt—NaCl—the Na+ ions would stick to the cathode and the Cl- ions would stick to the anode.) The charged electrodes would arrest the ions but allow the water to flow through, thereby reducing the ion concentration of the water. 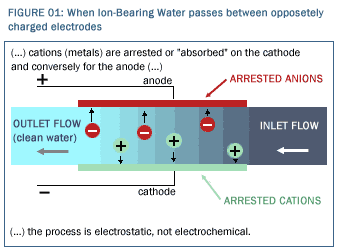 Therein lies the basic idea—arrest the ions while letting the water flow through the device, thereby selectively pulling the ions from the water stream and arresting them on the sides. The outflow has lower ion concentration than the inflow, meaning the water is purified by direct ion removal. Therein lies the basic idea—arrest the ions while letting the water flow through the device, thereby selectively pulling the ions from the water stream and arresting them on the sides. The outflow has lower ion concentration than the inflow, meaning the water is purified by direct ion removal.
CDI is not a chemical (oxidation/reduction) reaction but rather a simple electrostatic attraction process whereby charged ions "lightly stick" to the electrodes due to simple electrostatic attraction ("positive is attracted to negative"). In the vicinity of each electrode, as shown in Figure 2, ions of opposite charge concentrate in a layer extending outward a short distance from each charged electrode. The thickness of this ion layer is directly dependent on the magnitude of charge applied to the surface of the electrode. The charged electrode (charged positively in the diagram) causes the counter ions (charged negatively in the figure) to dehydrate and "lightly stick" to the electrode surface. No electrons are actually transferred to ions at either electrode surface, and because that is the case, the process is energy-inexpensive (<<1 watt-hour per liter of water) for removing ions from water. Electron transfer (oxidation/reduction) is expensive and energy intensive, but electrostatic attraction is downright cheap. Obviously, larger electrode surface areas arrest more ions from the water than smaller surface areas—the number of ions that can be attracted is proportional to surface area presented by the charged electrode to the fluid. That is why the ultra high surface area of Reticle Carbon© is so attractive—it allows colossally high electrode surfaces to be presented to the fluid. Normal materials (i.e., smooth materials) have approximately one square inch of surface area contacting the fluid for every square inch of cross section of the electrode. With normal materials, the 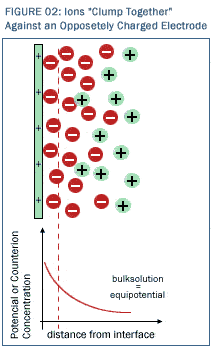 surface area of the material IS the cross sectional area. For such materials, the number of ions that could be arrested will be small. Reticle Carbon© is a special material, for it has many thousands of square inches of solid-to-fluid surface area for every square inch of cross section of the material. The surface of the material is pitted and fissured like craters on the moon so that the actual solid-to-liquid contact area is astronomical. With such materials in which one square centimeter of cross section contains many thousands of square centimeters of solid-liquid surface contact, the number of ions that can be arrested is far larger. Clearly, building a cost-effective CDI ion adsorber is very much a "surface area game"--the higher the surface area the solid presents to the fluid, the higher the scale at which the electrostatic process can be made to operate. Reticle Carbon© offers surface areas heretofore unimagined. surface area of the material IS the cross sectional area. For such materials, the number of ions that could be arrested will be small. Reticle Carbon© is a special material, for it has many thousands of square inches of solid-to-fluid surface area for every square inch of cross section of the material. The surface of the material is pitted and fissured like craters on the moon so that the actual solid-to-liquid contact area is astronomical. With such materials in which one square centimeter of cross section contains many thousands of square centimeters of solid-liquid surface contact, the number of ions that can be arrested is far larger. Clearly, building a cost-effective CDI ion adsorber is very much a "surface area game"--the higher the surface area the solid presents to the fluid, the higher the scale at which the electrostatic process can be made to operate. Reticle Carbon© offers surface areas heretofore unimagined.
To make matters even better, Reticle Carbon© electrodes can be built at a fraction of the cost of previously available electrode materials, and an even distribution of charge can be maintained throughout the entire Reticle Carbon© mass and therefore across the entire Reticle Carbon© surface because it is monolithic (single solid mass) and highly conductive. All we have to do is attach a wire to one edge of an electrode-shaped mass of Reticle Carbon©, apply a charge to the wire, and that charge is conducted immediately throughout the entire solid Reticle Carbon© mass. This means that we can charge an electrode with surface area the size of Hoover Dam by using a mere 60 gram mass of Reticle Carbon© and hooking it up to a wire on its edge! That mass will have virtually zero energy consumption (because the ion adsorption process is electrostatic and does not transfer electrons). The adsorptive ability of such a surface area is literally incomprehensible. In order to create a commercial scale CDI system, one simply "sandwiches" together a system of Reticle Carbon© electrodes in parallel flow channels as shown in Figure 3. 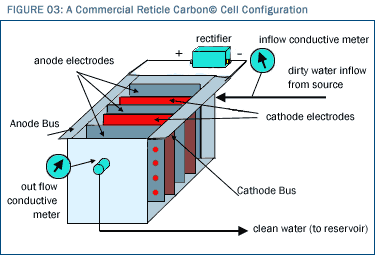 In the figure, ion-bearing solution enters at the upper right, flows through the unit leftward through the uppermost channel, rightward through the next channel down, leftward through the next channel down, and so on and so forth along a serpentine path all the way through the unit until clean water exits at the lower right. At every point during its journey through the cell, the solution is exposed to colossally high surface area, oppositely charged Reticle Carbon© electrodes that line the sides of all channels. As this occurs, ions are continuously pulled out of the water throughout its journey and adsorbed on the sides, leaving nothing but clean water to flow out the bottom. The magnitude of fluid-surface interface is astronomical with this type of configuration. The water is thereby efficiently and effectively purified as it traverses the set of channels that comprise the cell. (Cells of this serpentine type are commercially available from a number of vendors. All we need to do is populate them with Reticle Carbon©. We have also designed, constructed and operated our own cells, as well.) When, after processing a significant volume of ionized water, the Reticle Carbon© is completely covered with ions, the highly adsorbed electrodes must be flushed and cleaned for another round of use. Such flushing is accomplished through a surprisingly simple method—by simply grounding the electrodes and eliminating their net charge to drive off the adsorbed ions electrostatically, i.e., to drive them back into the fluid that is then resident within the chamber. Make no mistake, electrostatic repulsion is the natural state of affairs as shown in Figure 4, and removal of the charge from the electrode means that the adsorbed layer strives intrinsically to drive all the ions back into solution. 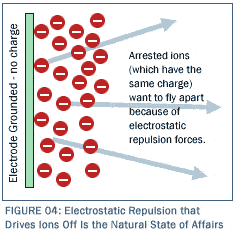
In order to accelerate the process of desorption, it is easily possible to reverse the polarity(and in fact to leave them permanently. Such polarity reversal, which is illustrated in Figure 5, should dramatically accelerate the desorption of ions from the electrodes. 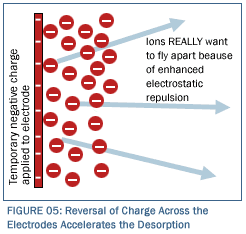
When such elimination of charge occurs, all the previously adsorbed ions are desorbed back into the fluid that is then resident within the chamber, which is a wastewater fluid. This then-resident fluid then serves as a working fluid to remove the ions from the chamber and deposit them in concentrated form somewhere else. Grounding of the electrodes with consequent desorption back into solution (which we call regeneration) flushes the ions that have been adsorbed off of the electrodes and reinitializes those electrodes for another round of use. To some, regeneration seems an arcane or esoteric process. However, there is nothing complex or counterintuitive about it at all. It is simple and natural. Desorption is the natural state of affairs, and it is the state of affairs that occurs when the cell is turned off. It is well to consider that the concentrated fluid that results from the electrode regeneration process will contain substantially higher concentration of ions than does the inlet water. For instance, if inlet water contained 100 parts per billion of total dissolved solids (including arsenate, arsenite, sulfate, carbonate, iron, magnesium, etc.), a significantly larger quantity of water would be purified and delivered to commercial use before the activated carbon electrode is saturated than would be the case for say brackish inlet water that contained 1,500 ppm salt concentration. While the regeneration process will effectively make water that ranges between 3 to 1000 times more concentrated than the feed inlet concentration, it should be noted that the final concentration of the regenerated solution will be consistent because it depends on the absolute number of ions that can be adsorbed on the electrode surfaces and the absolute volume of water resident within the chamber at the time of regeneration. In other words, the total number of ions per unit volume of working fluid will be roughly the same no matter what inlet water is treated. This makes treating the discharge solution much easier to handle—its concentration is predictable and consistent. It is well to emphasize that the high concentration ion solution produced during regeneration may have economic value. Arsenic itself is used in pesticides, in treated lumber, and in some semiconductor chip manufacturing. Selenium is used in xerography. Gold, silver, and palladium are precious metals. If the economic value of the concentrate solution is high enough to warrant recovery, a recovery plant could be constructed to recover ions from solution as a precipitate or as a metal. (If a recovery plant already exists at a site, so much the better.) The regeneration solution would be an excellent, consistent feedstock for a recovery plant. (If one wanted to concentrate the concentrate even further, one could run the concentrate through a second independent stage of Reticle Carbon© CDI or even a RO process, creating a further order of magnitude or more concentration. This could be continued until some form of final recovery were initiated.) If the concentrated solution recovery economics are not favorable, the solutions can be placed in holding ponds to allow for evaporation and then treated with ferric sulfate or other precipitating agents to ensure that pernicious ions such as arsenic, lead, mercury, or selenium are rendered stationary and inert. The sludge can be collected and shipped to disposal areas. The water that is filtered from such sludge could be recycled through the plant to process that water a second time. It should be noted that the volume of water in the concentrate portion will be only 1/3 to 1/1000 the total flow through the Reticle Carbon© CDI deionization units over the period of time it takes to load the electrodes, so recycling it through the cells would create a relatively small water inventory for the process. This is an important insight—the astronomical volume shrinkage leading to the first stage concentrate itself creates a very large quantity of ion free water. The volume differentials are very much in favor of massive water purification through arsenic removal. It is important to note that the voltages used in Reticle Carbon© CDI cells can be lower than the oxidation potential of water, i.e., lower than 3 volts. For voltages higher than the oxidation potential of water, the Reticle Carbon© CDI cell would be producing oxygen and hydrogen at the anode and cathode and thereby would be "wasting" energy. The Reticle Carbon© CDI cells will operate in the range of 2 volts. Furthermore, it is clear that the Reticle Carbon© CDI cell is a direct current (DC) device, not an alternating current (AC) device. Reticle Carbon© CDI is a low voltage, high amperage DC device. Interestingly, that is precisely the voltage that is produced by a state of the art solar photovoltaic device. That means that the Reticle Carbon© CDI cell is ideally suited to being sized to and coupled with a photovoltaic device for "remote from the power grid" ion removal (e.g., hardness removal, desalination, toxic ion removal). The only issue with regard to remote water production would be to be able to accomplish the low head pumping with DC rather than AC pumps operating at 2-3 volts. Given the modest pumping requirements, this should be extremely easy and economically attractive. Returning to Figure 1, it is clear that CDI is nondiscriminating with regard to which ions it removes. It removes all charged ions without discrimination. It "cleans house." It may remove +3 ions differentially faster than +1 ions and similarly for –3 and –1 ions, but it removes all charged ions from the bulk solution. For example, it softens the water (by removing calcium, carbonate, and sulfate ions) at the same time as it removes arsenic, selenium, and salt ionic species. Reticle Carbon CDI takes all the ions out of the water in one integrated mass, thereby deionizing the water entirely. |
|
copyright reticle 2003 webdesign beatriz alentejano |
|