
|
|
|
|
|
|
|
|
 |
|
|
|
||||
|
|
|
||||
     |
The Previously Existing (Pre-Reticle) State-of-the-Art for Carbon Electrode Materials Why has no one duplicated the properties of Reticle Carbon©? Why hasn’t CDI achieved the efficiency claimed for Reticle Carbon©? To understand the history and the parameters of the pre-Reticle best in class, one must begin by understanding that carbon occurs in numerous crystalline forms. The most common form of carbon is a hexagonal platelet known as a graphine plate. Graphite and activated carbon are comprised of such graphine plates. Both are electrically conductive materials with relatively low densities. The key distinction between graphite and activated carbon lies in the arrangement of graphine plates. The graphine plates within graphite are aligned in sheets with high crystallographic order; graphite is in fact a crystalline material. In sharp contrast, while activated carbon is composed of the same elemental graphine plates, in activated carbon, they are arranged entirely at random. There is no regular, intrinsic structure or organization of the plates. Activated carbon is not a crystalline material. More specifically, activated carbon exhibits short-range order (the order implicit in the graphine planes themselves), but absolutely no long-range order (completely random arrangement among the graphine plates). By contrast, graphite exhibits short-range order and long-range order together. Because of the completely random order among the graphine plates within activated carbon (as contrasted with the crystalline order in graphite), activated carbon exhibits much higher intrinsic surface area than does graphite. Figure 1 illustrates how this occurs. In effect, there is a huge amount of unused space in and around the randomly arranged plates, just as there is a lot of space in a bowl of randomly organized Corn Flakes. The resulting surface area difference between graphite and activated carbon is profoundly significant for the Reticle Carbon© electrode material. The Reticle Carbon© fabrication process is able to preserve the much higher surface area in the originating material (activated carbon) than processes that would begin with other carbon precursor materials, thereby transferring that much higher activated carbon surface area directly into the high surface area of finished, monolithic electrodes. Activated carbon is manufactured from natural precursor materials, such as coconut shells, 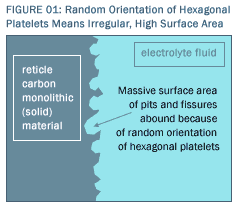 wood, sawdust, petroleum, etc. The precursor carbonaceous material is initially carbonized at high temperature in an anaerobic atmosphere. The aromatic rings of the original material are converted into graphine plates while the other organics are volatilized and driven off. Because natural materials are not ordered, the residual carbonized material will have randomly ordered graphine platelets (distinguishing it from graphite which has ordered plates). The carbonized material will have some measure of porosity; however, the porosity in the material can be dramatically increased through an activation process. The carbonized material is heated and reacted with steam or chlorine, which reacts to volatilize some of the surface graphine plates, leaving networks of pores in the material. These pores account for the large surface areas. It is this highly randomized, highly porous material that initiates the Reticle Carbon© and other manufacturing processes. wood, sawdust, petroleum, etc. The precursor carbonaceous material is initially carbonized at high temperature in an anaerobic atmosphere. The aromatic rings of the original material are converted into graphine plates while the other organics are volatilized and driven off. Because natural materials are not ordered, the residual carbonized material will have randomly ordered graphine platelets (distinguishing it from graphite which has ordered plates). The carbonized material will have some measure of porosity; however, the porosity in the material can be dramatically increased through an activation process. The carbonized material is heated and reacted with steam or chlorine, which reacts to volatilize some of the surface graphine plates, leaving networks of pores in the material. These pores account for the large surface areas. It is this highly randomized, highly porous material that initiates the Reticle Carbon© and other manufacturing processes.
Binder Carbon
The early attempts to create activated carbon monoliths used "binders." In effect, people attempted to "glue" together the activated carbon particles and hope that the glue did not occlude or "gum up" too much space or thwart too much of the original activated carbon surface area. In the early prototype activated carbon electrode implementations, granulated activated carbon particles were held together under pressure, but these electrodes proved to be bulky and potentially explosive. Subsequent technology was characterized by blending carbon particles with a variety of organic or inorganic binders. Essentially, the carbon particles are "glued together with model airplane glue." However, the disadvantages of the binder approach are immediately apparent. The binders reduce the electrode conductivity, and importantly they occlude pore spaces that are intrinsic in the activated carbon. Additionally, binders have proven to be chemically unstable and amenable to breakdown. Binder carbon electrodes disintegrate over time as the binder deteriorates. Binders have actually proven to deteriorate at rates faster than people like, rendering binder carbon electrodes uneconomic. Electrodes made using binders to amass activated carbon particles have never demonstrated surface areas in commercial practice that exceed 400 m2/g, and they turn to "oatmeal" as the binder deteriorates with use.
The most recent carbon electrode fabrication processes that predate Reticle have focused on carbonizing more complex carbonaceous materials than the typical precursors to activated carbon. For example, people have been carbonizing phenolic resins or other organic precursors to produce activated carbon electrodes. Unfortunately, those electrodes have demonstrated only slightly higher surface area than the binder carbon electrodes. For example, electrodes based on carbonizing phenolic resins or other organic precursors have resulted in surface areas only marginally higher than binder carbon and much more expensive because of the high cost of the precursor carbonaceous materials. We understand that such carbons claim surface areas as high as 800 m2/g, but no commercial shipment has been measured at over 400 m2/g as Reticle understands it. This is the same surface area as binder carbon. The resin carbonization processes, known through tradenames such as Aerogels and Xerogels, are considered by many to be state-of-the-art.
Until the emergence of Reticle Carbon©, because of the complex and expensive progress with binder or carbonized electrode materials, enthusiasm for and production of activated carbon electrodes and for CDI waned and became stagnant. People have become frustrated and looked elsewhere for the solution to the water deionization and desalination problem (e.g., RO) or have simply done without. Indeed, some have gone so far as to abandon activated carbon electrodes altogether in favor of devilishly complex carbon-metal composite electrodes (which themselves have proven costly yet just as ineffectual as what they aspire to replace). It is doubtful that they will ever provide the sought-after answer. Prior to the emergence of Reticle Carbon©, the situation was becoming dire and pessimistic in the CDI arena. Reticle Inc. has developed and patented a much improved manufacturing process for making activated carbon electrode material and retaining the desirable properties of the precursor activated carbon, particularly the colossal surface area and the conductivity. In particular, the Reticle process fabricates monolithic carbon material that is ideally suited for use as CDI electrodes by electrically and physically interconnecting granulated activated carbon particles using a new method. Because the Reticle process begins with activated carbon particles and preserves most of their desirable properties within the monoliths we produce (particularly the surface area and the conductivity), it is obvious that we must strive to select feed material with an eye toward maximizing the active adsorption area therein. The Reticle process (which will not be disclosed here) is able to preserve in the fabricated electrode material a much higher proportion of the original surface area that was in the feed material than the best binder carbon and the best carbonization-of-complex-precursors process. |
|
copyright reticle 2003 webdesign beatriz alentejano |
|