
|




|

|

Reticle Carbon Technology used in water purification
A critically important use of Reticle Carbon technology is based on a simple, proven technology called "capacitive deionization (CDI)," which works as follows. If two oppositely charged electrodes are placed in a tank containing ionized water as illustrated in Figure 1, the anionic species will seek and adsorb on the anode and the cationic species will seek and adsorb on the cathode. If ion-bearing water were passed between the electrodes as shown in the figure, the ions would adsorb on the respective electrodes (i.e., they would be arrested by electrostatic attraction on the electrodes), and the net outflow would be water with lower ion concentration than the water that entered at the inlet. In effect, the charged electrodes would have arrested the ions but allowed the water to flow through, thereby reducing the ion concentration of the outlet water. Therein lies the basic idea—arrest the ions while letting the water flow through the device, thereby deconcentrating the ions in the water that is flowing through.
CDI is not a chemical (oxidation/reduction) reaction but rather a simple electrostatic attraction process whereby charged ions "stick" naturally due to electrostatic attraction to immersed charged electrodes. Ions of opposite charge (counter ions) concentrate in a layer extending outward a short distance from each charged electrode (the positively charged electrode or anode). The "thickness" of this layer is directly dependent on the potential applied to the surface of the charged electrode. The charged electrode (charged positively in the diagram) causes the counter ions (charged negatively in the figure) to dehydrate and adsorb on the electrode surface. In the vernacular, ions merely "stick" to the charged electrodes; no electrons are actually transferred. Because no electrons are transferred, capacitive deionization (CDI) is energy-inexpensive (<1 watt-hour per liter of water) for removing ions from water.
Once the electrodes are completely covered with ions after processing a significant volume of ion-bearing water, the highly adsorbed ions must be detached from the electrodes and flushed from the system. This is accomplished in a quite trivial fashion—by simply grounding those electrodes, eliminating their net charge, and thereby releasing the adsorbed ions to the water stream then resident within the cell, which serves as a working fluid to remove them from the chamber and deposit them in highly concentrated form somewhere else. Grounding of the electrodes with consequent desorption back into solution (which we call regeneration) flushes the ions that have been adsorbed on the electrodes and reinitializes the two electrodes for more use.
It is obvious that larger electrode surface areas adsorb more ions from the water than smaller surface areas—adsorptivity is proportional to surface area presented by the charged solid to the fluid. It is also obviously a function of the conductivity of the solid and thus its ability to deliver charge to the surface. The net volume of ions that is adsorbed will be dependent on the available charged surface area, the magnitude of charge, and the thickness of the ion-layer. Not only should the available surface area be maximum, an even distribution of charge across the surface should be maintained. More electrode surface area means more ions adsorbed and therefore more volume of water deionized before the electrodes need to be regenerated.
Among the electrode materials that have sufficiently high conductivities, CDI developers have come to realize that they have to employ the largest adsorption surface area materials they can possibly find. Activated carbon is one of the most obvious places to look for ultra high surface area electrode material. Activated carbon offers surface areas that are intrinsically much larger than other materials and are as conductive as many metals. However, until Reticle Carbon, they are powdered rather than solid. Today, powdered activated carbon is commercially available with surface areas that range from 400 m2/g to as high as 3000 m2/g. If monolithic (i.e., solid) electrodes could be manufactured from such materials (if the intrinsic surface areas of those materials could be preserved through an electrode fabrication process), those electrodes would provide colossally high surface areas and would therefore offer exceptional properties for ion removal. That in a nutshell is what Reticle Carbon provides—ultra high surface area, monolithic (i.e., rigid), conductive, chemically inert electrode material that is manufactured directly from and takes its properties from activated carbon. Reticle Carbon can achieve over 2000 m2/g in monolithic electrodes, far higher than the 400 m2/g that is the current "best in class" in the industry.
In order to manufacture a commercial electrochemical cell, one merely configures alternating charged electrodes very simply as in Figure 3. "Sandwiched," serpentine flow design cells are available today commercially at very modest costs. In fact, they are purchased as bathtub like containers that are populated with alternatively charged electrodes to which electrode surfaces can be readily attached. There is no mystery in how to design and procure serpentine flow electrochemical cells and how to insert Reticle Carbon electrodes into such cells, replacing the electrodes that were originally there. The essence of the Reticle Carbon technology is to manufacture the specialty Reticle Carbon to attached to electrodes already resident within standard electrochemical cells of the form in Figure 3 and thereafter to place the resulting Reticle Carbon-populated cells into service to remove ions from water.
In the commercial configuration, we place conductivity meters at the cell inlet and outlet to measure the ion content of the inbound and outbound water. 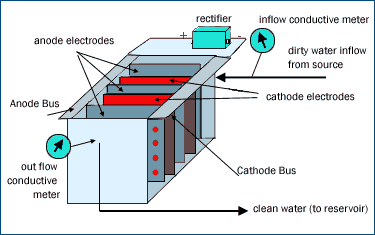 Those inbound and outbound conductivity measurements are used to control the unit. There is a serpentine flow of water between alternatively charged electrodes. The charge across the electrodes is created and maintained by a rectifier that maintains a constant, controlled voltage. (A constant, controlled voltage is important, for we do not want the voltage to climb above the decomposition potential of water.) The electrodes themselves are created by "mosaicing" solid, monolithic Reticle Carbon wafers to conventional stainless steel or other electrodes that come with standard order electrochemical cells. The components of the Reticle Carbon cell are very simple and very conventional. The "secret sauce" in the Reticle Carbon cell is the ultra high surface area Reticle Carbon itself. The entire cell is conventional and standard save for the ultra high surface area used to line the electrodes and create colossal surface areas. Those inbound and outbound conductivity measurements are used to control the unit. There is a serpentine flow of water between alternatively charged electrodes. The charge across the electrodes is created and maintained by a rectifier that maintains a constant, controlled voltage. (A constant, controlled voltage is important, for we do not want the voltage to climb above the decomposition potential of water.) The electrodes themselves are created by "mosaicing" solid, monolithic Reticle Carbon wafers to conventional stainless steel or other electrodes that come with standard order electrochemical cells. The components of the Reticle Carbon cell are very simple and very conventional. The "secret sauce" in the Reticle Carbon cell is the ultra high surface area Reticle Carbon itself. The entire cell is conventional and standard save for the ultra high surface area used to line the electrodes and create colossal surface areas.
There is one other salient observation that merits careful discussion and consideration. When the charged plates are immersed in the liquid as in the foregoing diagrams, the Reticle Carbon cell becomes in effect a capacitor. Negatively charged ions in the bulk fluid gravitate toward the cathode, thereby offsetting or neutralizing the charge on the cathode by forming an electrostatically offsetting Stern layer in the vicinity of the cathode. Similarly, positively charged ions in the bulk fluid gravitate toward the anode, thereby offsetting the charge on the anode by forming an electrostatically offsetting Stern layer in the vicinity of the anode. As such, the "fully charged" Reticle Carbon cell is in effect a very large capacitor. It contains Stern layer positive charge on the cathode and Stern layer negative charge on the anode. If the electrodes were instantaneously grounded, that large positive charge on what used to be the cathode and that large negative charge on what used to be the anode would instantaneously represent a very large charge differential. That very large charge differential would discharge just like a capacitor. The cell therefore IS in a very fundamental sense a capacitor, and the capacity of the cell to adsorb ions is directly related to the capacitance of the cell. As we shall see, the capacitance of a Reticle Carbon electrochemical cell is very high because of the high surface area of the Reticle Carbon electrodes, in fact, startlingly so.
|

|

